ESR1 resistance mutations – What is the best technology for your patients?
Summary
Breast cancer is one of the most common types of cancer affecting women worldwide. In hormone receptor-positive, HER2-negative metastatic breast cancer, endocrine treatment has led to significant improvement in patient’s condition. However, tumors can develop resistance mechanisms throughout the therapy, for example via activating mutations in ESR1.
Molecular testing of ESR1 mutations via liquid biopsy next-generation sequencing (NGS) allows for non-invasive, real-time assessment of the large genomic regions of interest. Due to its ability to detect non-hotspot mutations as well as its recent improvement in sensitivity due to new molecular barcoding technologies, NGS is the method of choice to unbiasedly select patient ESR1 mutant patient for new selective Estrogen receptor degrader (SERD) treatment that has proven efficient to overcome this resistance mechanism and improve patient’s outcome
ESR1 Mutations and Breast Cancer
ESR1, the gene that encodes the estrogen receptor alpha (ERα), plays a crucial role in hormone-sensitive breast cancer. Patients suffering from hormone-sensitive, HER2-negative breast cancer can benefit from endocrine therapies such as aromatase inhibitor and selective estrogen receptor modulators. However, mutations in the ligand-binding domain of ESR1 can develop over the course of the treatment and lead to constitutive activation of the estrogen receptor, rendering the tumor resistant to endocrine therapies1. In this case, new, SERD-based treatment options can be explored to overcome the resistance2.
Liquid Biopsy NGS for Detection of ESR1 Mutations
Liquid biopsy, a minimally invasive diagnostic technique that involves analyzing circulating analytes in blood samples, has emerged as a revolutionary tool in the field of cancer diagnostics. By detecting and monitoring genetic alterations present in circulating tumor DNA, liquid biopsy NGS provides a comprehensive and real-time assessment of tumor evolution and treatment response (Figure 1).
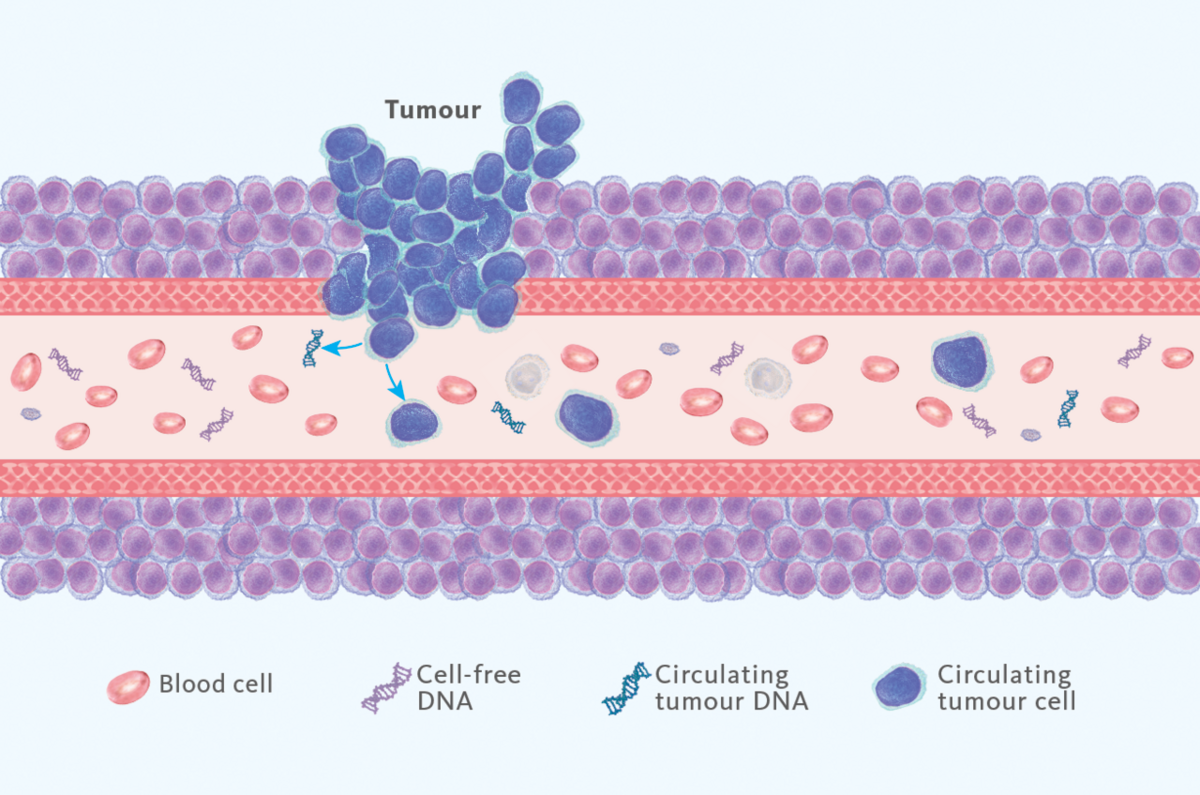
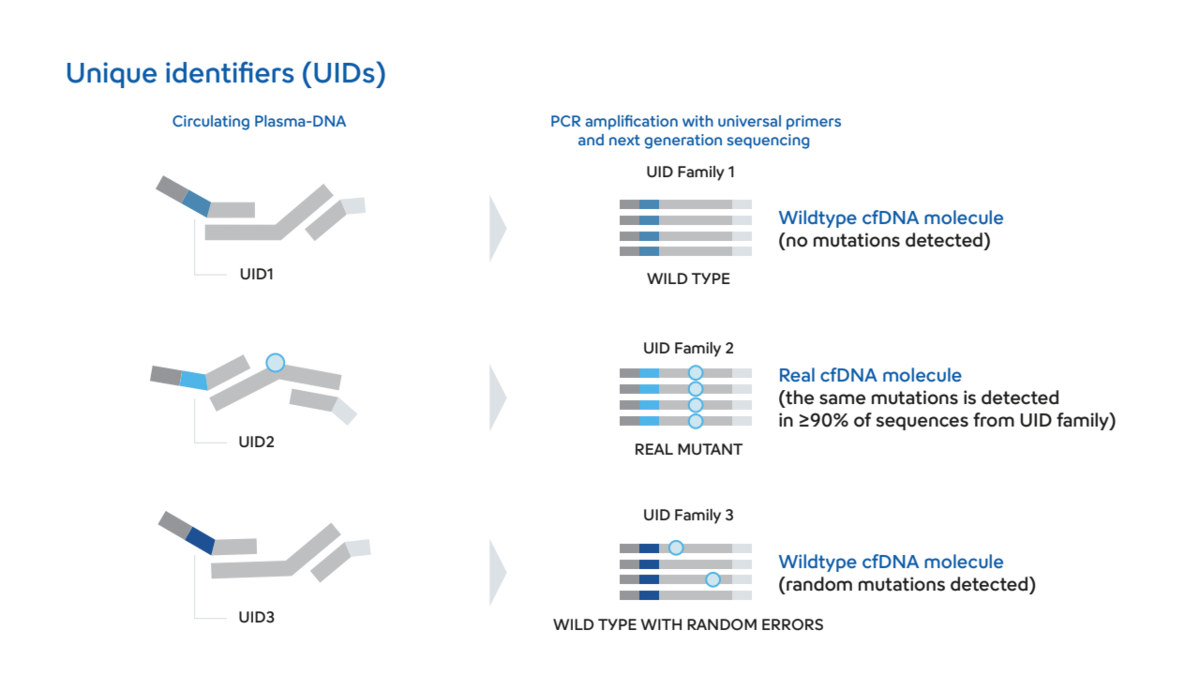
The application of liquid biopsy NGS for the detection of ESR1 mutations in breast cancer patients offers several key advantages. As ESR1 resistance mutations are generally developing during endocrine therapy, their detection requires regular follow up with new material, which appears too invasive in the context of classic tissue biopsy3,4. On the other hand, liquid biopsy only requires regular blood sampling to assess ESR1 mutational status in real-time, making it the first-choice method for ESR1 resistance mutation detection according to ASCO guidelines5. Moreover, NGS is particularly useful in these conditions as new barcoding technologies (or Unique identifiers) have drastically improved variant detection sensitivity (Figure 2). As a result, NGS assays can now be 50X-100X more sensitive than qPCR assays (similarly to ddPCR) while testing way larger genomic regions in parallel.
Sysmex’s Plasma-Seq Sensei breast cancer NGS solution
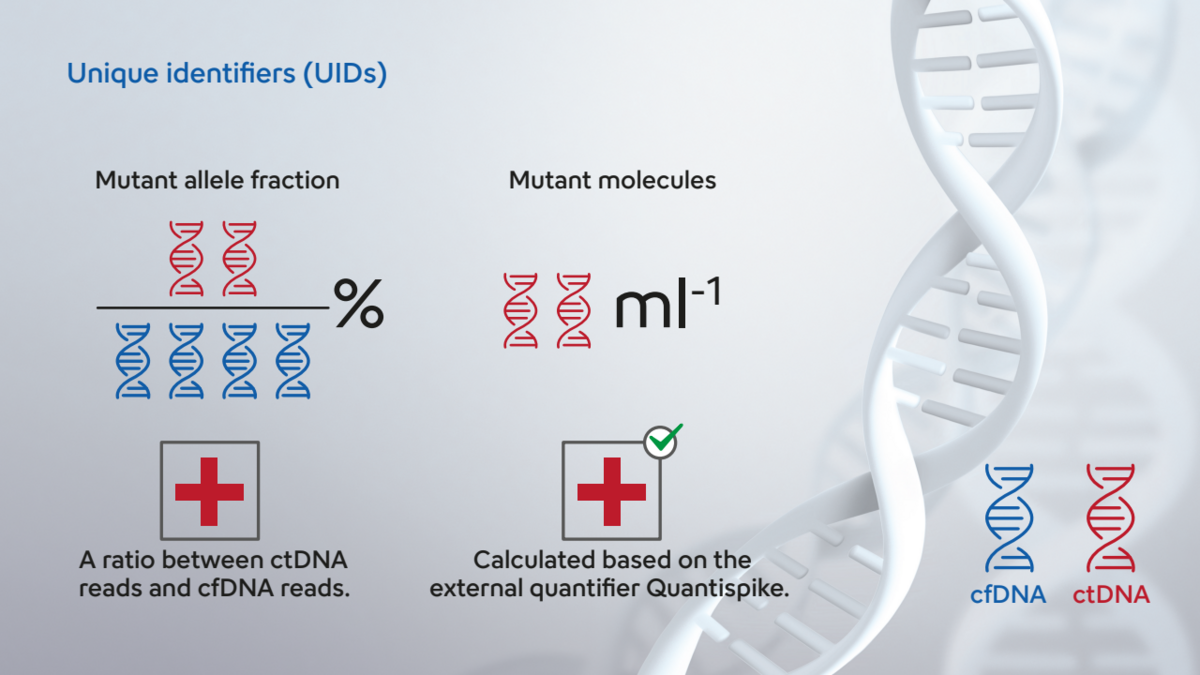
Sysmex is a leader in diagnostic testing. The Plasma-Seq Sensei breast cancer IVD kit is a CE-IVD labelled NGS library preparation kit designed to ease neoadjuvant and adjuvant therapies monitoring, early recurrence detection and resistance mutation detection including in ESR1 and PIK3CA genes. The kit is based on the Safe-Seq technology6, which uses molecular barcodes to improve ctDNA detection and allows Plasma-Seq Sensei to reach a sensitivity of 0.06% MAF. Moreover, our library preparation process includes a spike-in DNA called Quantispike allowing the quantification of absolute number of ctDNA molecules carrying specific mutations. Absolute quantification in ctDNA-based liquid biopsy has proven particularly useful, ruling out false negative arising from cfDNA release variation upon patient’s physical activity7,8, infection9 or even circadian cycle10,11 (figure 3). Using absolute quantification, Plasma-Seq Sensei can detect as little as 6 circulating mutant molecules in a background of 10’000 molecules, making sure no patients are left behind that could benefit from SERD treatment.
Future Directions and Implications
As precision medicine continues to shape the landscape of cancer care, the role of molecular testing in guiding treatment decisions is becoming increasingly crucial. In the realm of breast cancer, the detection of ESR1 mutations through liquid biopsy NGS solutions such as the Plasma-Seq Sensei breast cancer IVD kit represents a significant advancement that has the potential to improve patient outcomes and quality of life. Firstly, through definition of patient therapy, for example in case of ESR1 or PIK3CA resistance mutations. Secondly, via the upcoming development of clinical application of ctDNA-based testing, including (neo-)adjuvant therapy monitoring and early recurrence detection.
Author: Sysmex Suisse AG
References
[1] Brett, J. O., Spring, L. M., Bardia, A. & Wander, S. A. (2021): ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res. 23, 85
[2] Bidard, F.-C. et al. (2022): Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J. Clin. Oncol. 40, 3246–3256
[3] Toy, W. et al. (2013): ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat. Genet. 45, 1439–1445
[4] Robinson, D. R. et al. (2013): Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat. Genet. 45, 1446–1451
[5] Burstein, H. J., DeMichele, A., Somerfield, M. R., Henry, N. L. (2023): & for the Biomarker Testing and Endocrine and Targeted Therapy in Metastatic Breast Cancer Expert Panels. Testing for ESR1 Mutations to Guide Therapy for Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer : ASCO Guideline Rapid Recommendation Update. J. Clin. Oncol. 41, 3423–3425
[6] Kinde, I., Wu, J., Papadopoulos, N., Kinzler, K. W. & Vogelstein (2011): B. Detection and quantification of rare mutations with massively parallel sequencing. Proc. Natl. Acad. Sci. 108, 9530–9535
[7] Velders, M. et al. (2014): Exercise is a potent stimulus for enhancing circulating DNase activity. Clin. Biochem. 47, 471–474
[8] Neuberger, E. W. I. et al. (2022): Physical activity specifically evokes release of cell-free DNA from granulocytes thereby affecting liquid biopsy. Clin. Epigenetics 14, 29
[9] Hovhannisyan, G., Harutyunyan, T., Aroutiounian, R. & Liehr, T. (2023): The Diagnostic, Prognostic, and Therapeutic Potential of Cell-Free DNA with a Special Focus on COVID-19 and Other Viral Infections. Int. J. Mol. Sci. 24, 14163
[10] Madsen, A. T., Hojbjerg, J. A., Sorensen, B. S. & Winther-Larsen, A. (2019): Day-to-day and within-day biological variation of cell-free DNA. eBioMedicine 49, 284–290
[11] Poulet, G. et al. (2023): Circadian rhythm and circulating cell-free DNA release on healthy subjects. Sci. Rep. 13, 21675