Advancing precision oncology: sensitive detection of ESR1 mutations in plasma
Key points
- Novel SERDs like elacestrant show promise in overcoming endocrine therapy resistance for ER+/HER2- patients with ESR1 mutations.
- Liquid biopsy offers a non-invasive method for detecting ESR1 mutations.
- Highly sensitive assays are essential for accurate mutation detection in plasma.
- The Plasma-SeqSensei™ Breast Cancer IVD Kit demonstrated exceptional sensitivity, detecting ESR1 mutations in the SensID ESR1 reference material down to 0.06% MAF.
Background
The majority (70%) of newly diagnosed breast cancer patients are characterized as the luminal type. For them, oestrogen receptor alpha (ERa), encoded by the ESR1 gene and its cognate ligand oestrogen are the major drivers of tumour development and disease progression. Hence, agents that impair ER signalling in ER-positive breast cancers such as cyclin-dependent kinase (CDK) inhibitors and selective estrogen receptor degraders (SERDs) represent highly successful targeted therapies that are widely used in both early breast cancer, as well as the metastatic setting (Rugo H., 2023).
Challenges in ER-Positive breast cancer treatment
The emergence of mutations impacting the estrogen receptor gene, ESR1, may cause resistance to endocrine therapy. While ESR1 mutations are rare in primary ER+ breast cancers, endocrine therapy-induced selective pressure increases the occurrence of ESR1 mutations (up to 55%) in ER+ recurrent breast cancer patients (Najim O. et al., 2019). These mutations lead to constitutive activation of the ER, which in turn drives disease progression (Dustin D. et al., 2019).
Novel therapeutic opportunities
Newly developed treatments, such as the SERD elacestrant, have shown promise in improving survival (median PFS of 8.6 months compared to 2.1 months with SoC) for patients with ESR1 mutations linked to early endocrine resistance. However, a reliable diagnostic technique is needed to accurately identify ESR1 mutations to make use of this therapeutic potential (Bardia A. et al., 2021).
Detection of ESR1 mutations in plasma
Recently, liquid biopsy emerged as a useful method for a reliable detection of ESR1 mutations in a longitudinal and non-invasive way. For example, in the phase I study of elacestrant (Bardia A. et al., 2021) eligible patients were identified based on their ESR1 mutational status, which was assessed exclusively from circulating tumor DNA (ctDNA), with mutations D538G, Y537S, L536H/P, E380Q and Y537N being the most prevalent. Notably, other studies have shown that ESR1 mutations can be identified from plasma 6.7 months before the disease’s progression, making it a predictor of resistance to conventional endocrine therapy (Brett J. et al., 2021).
Guidelines emphasize using liquid biopsy for ESR1 testing for ER+/HER2- in advanced breast cancer
- The European Society for Medical Oncology (ESMO) recommends the testing of ESR1 mutational status via ctDNA, reflecting the advantages of ctDNA, which include its non-invasive nature and ability to dynamically track changes in tumor DNA.
- Additionally, The American Society of Clinical Oncology (ASCO) endorses routine testing for ESR1 for the same patients, especially in regards todisease recurrence (Burstein H. et al, 2023). This recommendation underscores the importance of utilizing ctDNA to guide therapeutic choices and addressing resistance to conventional endocrine therapy.
High sensitivity matters
- Despite numerous advantages, liquid biopsy can be challenging since many cancer patients have only a small amount of ctDNA present in their blood (Pascual J. et al., 2022), making it hard to detect.
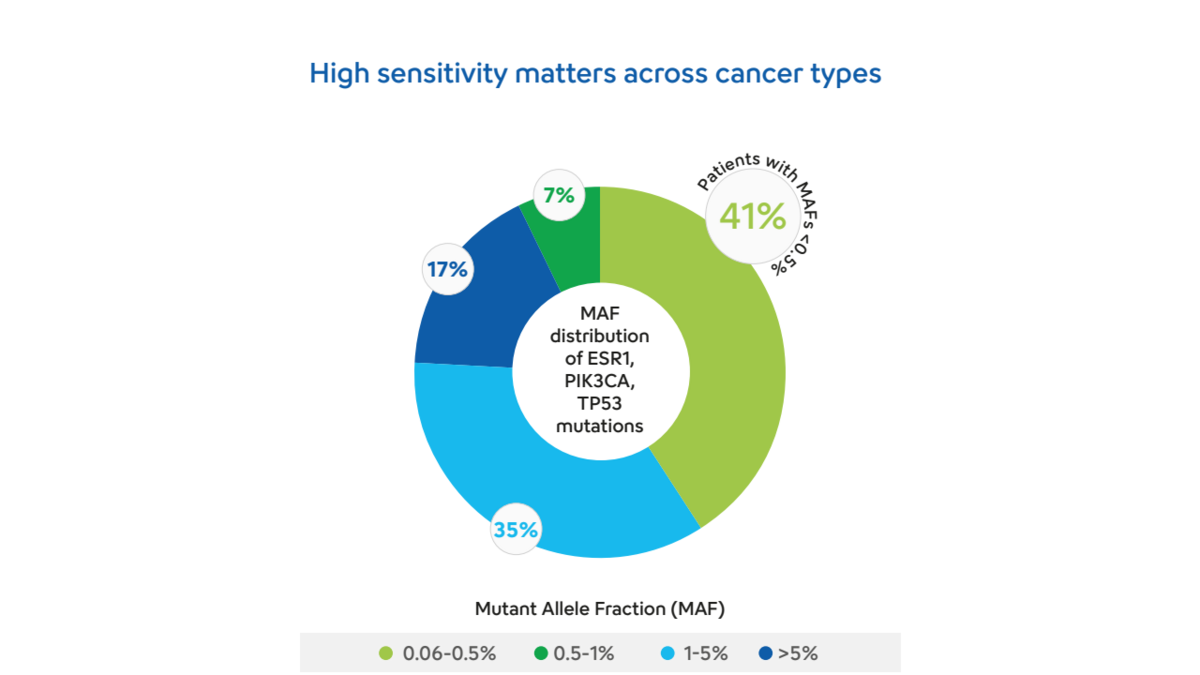
- Our recent study using the Plasma-SeqSensei™ Breast Cancer IVD Kit has shown that 48% of patients present ctDNA levels below 1% (Figure 1).
- Thus, showing that nearly half of metastatic ER+/HER2- breast cancer patients within the tested cohort (n=157) would have been misclassified as ctDNA negative if employed assays had sensitivity only down to 1% mutant allele fractions (MAFs).
- This further illustrates the need for sensitive liquid biopsy solutions that properly identify patients in need of therapy change.
Plasma-SeqSensei™ Breast Cancer IVD Kit
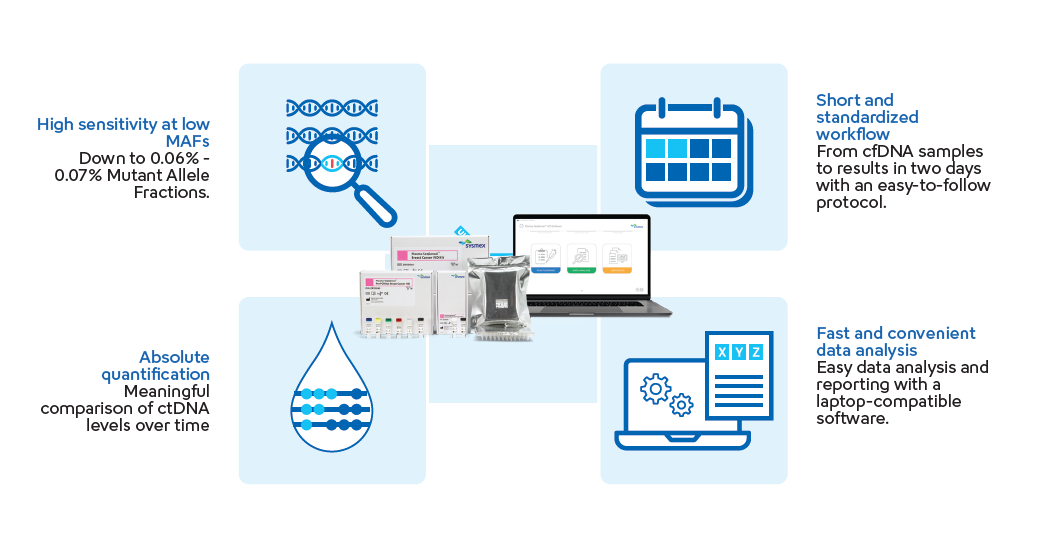
- The Plasma-SeqSensei™ Breast Cancer IVD Kit was created to enable highly sensitive and quantitative detection of mutations in ctDNA from the blood plasma of patients with breast cancer by utilising next-generation sequencing (NGS) technology.
- As underlined by the ESMO guidelines, sensitivity is key in reliable detection of ctDNA (Pascual
- J. et al., 2022). Plasma-SeqSensei™ offers the highest sensitivity of NGS-based liquid biopsy kits by detecting MAF as low as 0.06%, with a 95% confidence level, within a background of 10,000 wild-type copies.
- To ensure easy laboratory implementation and timely results, Plasma-SeqSensei’s™ short and standardised workflow delivers results within two days including the generation of easy-to-read reports using laptop-compatible Plasma-SeqSensei™ IVD Software.
Beyond ESR1, the Plasma-SeqSensei™ Breast Cancer IVD Kit covers key gene mutations in critical hotspots,
from PIK3CA, AKT1, ERBB2, KRAS and TP53, to detect established and emerging predictive markers, resistance mutations, and the most frequently occurring genetic alterations in breast cancer. This ensures a broad coverage across the population while also taking into account cost-effectiveness in the patient care.
Table 1: ESR1 regions detected by the Plasma-SeqSensei™ Breast Cancer IVD Kit. For complete panel’s coverage, please refer to the Instruction for Use
| Gene | Transcript* | Amino acid end | Most common ESR1 mutations |
| ESR1 | ENST00000440973 | 370 | E380Q |
| ESR1 | ENST00000440973 | 460 | S463P, L469V, F461V, L466Q |
| ESR1 | ENST00000440973 | 529 | D538G, Y537S/C/N/D, L536H N532K, V534E/L |
*Sequence source: Ensemble database
Plasma-SeqSensei™ Breast Cancer IVD Kit validation with sensID ESR1 reference set
The ability of the Plasma-SeqSensei™ Breast Cancer IVD Kit to detect low MAF mutations in the ESR1 gene was tested using serial dilution of the ESR1 Reference Set 1% AF cfDNA, which was kindly provided by SensID (SKU: SID-000144).
SensID’s ESR1 Reference Set 1% AF cfDNA is specifically designed as quality control material for lab developed tests (LDTs) and CE-IVD assays. The selection of ESR1 mutations (L536H, Y537C, L536P, Y537S, L536R, D538G, E380Q, S463P, and Y537N) was reviewed and endorsed by various experts in the field, representing the most prevalent ESR1 mutations (Grinshpun A. et al., 2023).
The Plasma-SeqSensei™ Breast Cancer IVD Kit is able to detect all nine ESR1 mutations included in SensID’s ESR1 Reference Set down to a limit of detection of 0.06% MAF. This high level of sensitivity allows for the detection of mutations at much lower MAFs compared to other liquid biopsy kits, which typically have a detection limit of 0.5% MAF.
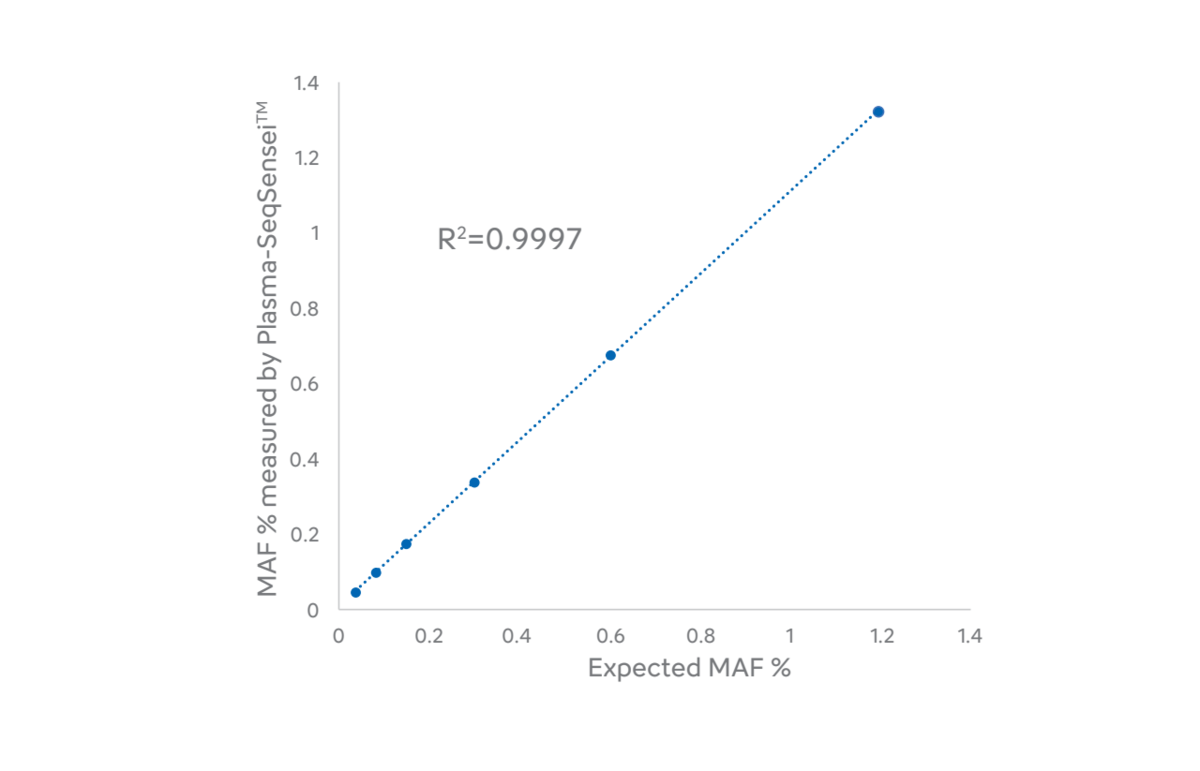
Furthermore, the regression analysis showed a high correlation (R2= 0.9997) between the expected values from SensID’s reference and the actual measurements for all nine ESR1 mutations (Figure 4). This powerful correlation demonstrates the exceptional accuracy of the Plasma-SeqSensei™ test.

Conclusion
ER+/HER2- advanced breast cancer poses a unique challenge for liquid biopsy due to low ctDNA levels in plasma. Our data revealed that nearly half of the samples exhibited ctDNA MAFs below 1%.
Therefore, highly sensitive liquid biopsy assays are necessary for the effective detection of clinically relevant mutations. The Plasma-SeqSensei™ Breast Cancer IVD Kit demonstrates exceptional sensitivity in this regard. Analysis using SensID reference material confirms its ability to robustly detect crucial ESR1 mutations even with a low MAF range of 1% to 0.06%. The approval of new Selective Estrogen Receptor Degraders (SERDs) marks a significant advancement in the treatment landscape for ER+/HER2- advanced breast cancer patients. Coupled with sensitive liquid biopsy, this remarkable new therapy ensures better treatment decisions, leading to improved patient survival.
Download White paper
